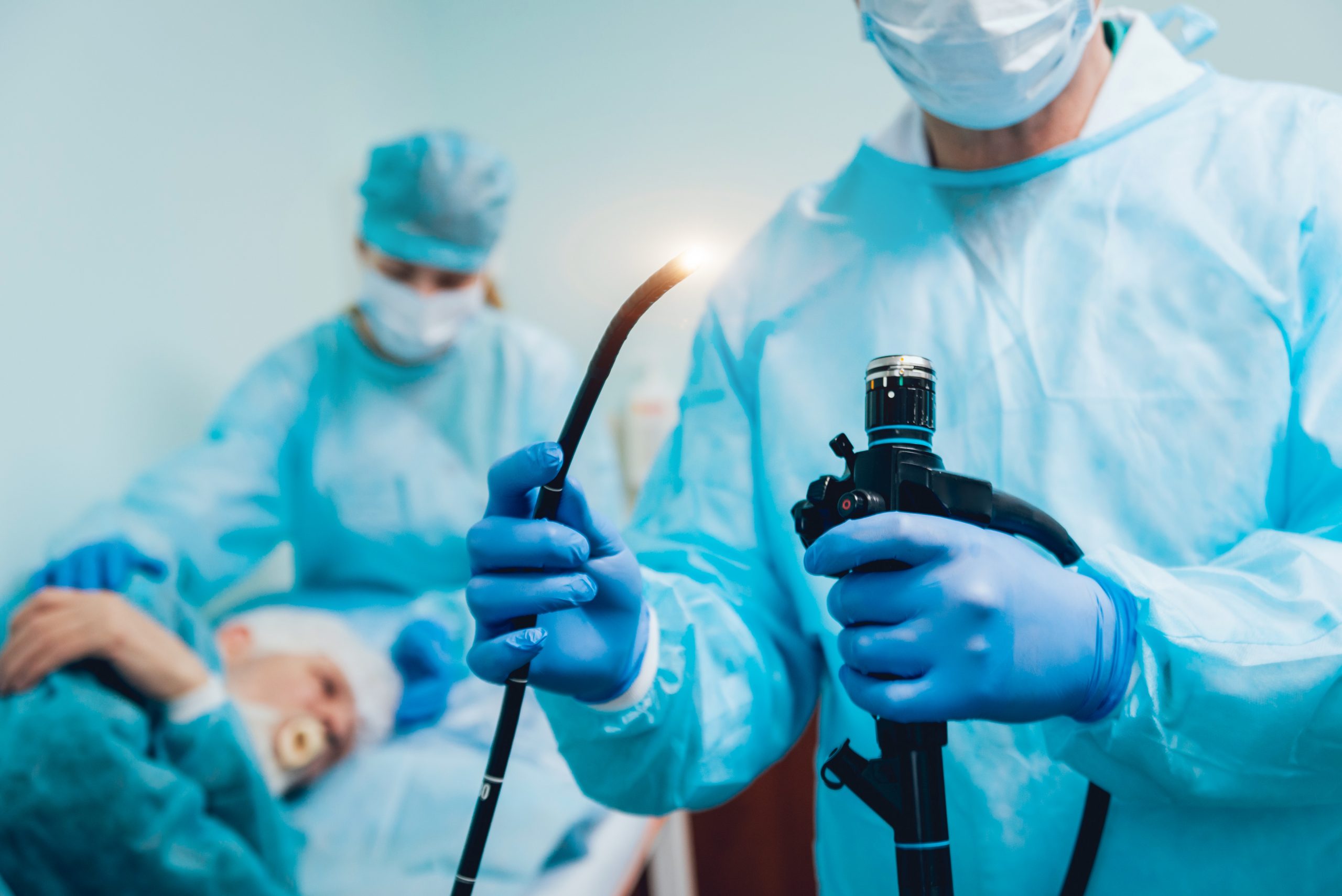
Cleaning Validation for Reusable Medical Devices
March 20, 2025
Anne M. Schuler
reusable medical devices, cleaning validations, reprocessing of reusable medical devices, end-of-life conditioning, AAMI ST98, ISO 17664, medical device IFU, protein analysis, hemoglobin analysis
End-of-life conditioning, 21 CFR 801, instruction for use development
Reusable medical devices are devices that health care providers can reprocess and reuse on multiple patients. If they are not properly reprocessed between use, there is a greater likelihood of microbial transmission and a high risk of infection. Cleaning is the physical removal of soil and contaminants, according to the FDA guidance document Reprocessing Medical Devices in Health Care Settings: Validation Methods and Labeling Guidance for Industry and Food and Drug Administration Staff effective cleaning should:
Therefore, cleaning methods must be validated separately from disinfection or sterilization processes which are validated to demonstrate appropriate microbicidal reduction. A reusable device cannot be effectively sterilized or disinfected unless it is properly cleaned first. Under FDA labeling regulations (21 CFR 801) manufacturers of reusable medical devices must provide written instructions for cleaning reusable devices between use, these cleaning processes must be thoroughly validated to confirm their effectiveness.
At LexaMed, we specialize in designing and executing cleaning validations tailored to each unique device following the guidance provided in the following documents:
Complex device designs may present particular challenges to cleaning and validation therefore our experienced project managers work closely with manufacturers to understand the device and its cleaning requirements in order to generate a protocol that incorporates all elements of the standards such as end-of-life conditioning, soil selection, test methods and acceptance criteria.
Through repeated use, the surface of a device may begin to deteriorate which could lead to soil accumulation therefore devices used for validation testing must be reflective of end-of-life conditions to represent worst case conditions for cleaning. In order to achieve this, prior to use in a validation study, devices undergo simulated use by exposure to a minimum of six (6) cleaning and sterilization or disinfection cycles to condition them for testing. Clients can either perform this step themselves or utilize LexaMed’s expertise.
Following end of life conditioning trained analysts inspect each device to determine the most difficult to clean area such as lumens, hinges and matted surfaces. Devices are then contaminated, focusing on these hard to clean areas, with an organic test soil material that contains clinically relevant components such as hemoglobin, protein, carbohydrates or endotoxin. The device may be soiled by handling with soiled gloved hands and/or complete immersion in the test soil to simulated worst case. Movable parts are actuated during soiling to simulate clinical use. The soil remains in place for a sufficient time to simulate time between patient use and cleaning.
Contaminated devices are cleaned using either the manufacturer’s recommended cleaning process or a process developed as part of the validation. Using the manufacturer’s specified detergent, cleaning is performed using a manual process such as wiping, brushing with a soft bristle brush, flushing, soaking or a combination of these methods. Cleaning may also be conducted using an automated process in a washer-decontaminator. Following cleaning, the devices are visually inspected for residual soil.
Cleaned devices, a negative control device which was cleaned without soiling and a positive control which was soiled but not cleaned are extracted in deionized water and the extracts analyzed by appropriate test methods to determine the level of challenge soils remaining. Test methods used cannot always recover 100% of the soils present on a device due to the various materials of construction and configuration. Therefore, prior to extraction a Recovery Efficiency Study is performed for each challenge soil to determine the percentage that can be recovered during extraction. Using the results of this test, a recovery factor is calculated and then applied to all testing conducted. Multiple extraction method attempts may be necessary to determine the most efficient method for soil removal.
The level of residual challenge organisms remaining following cleaning are then compared to the acceptable levels provided in AAMI ST98 to determine the effectiveness of the cleaning process.
The final step is an evaluation to determine if the cleaning process effectively removed detergent residuals to a safe non-toxic level. A cleaned device is subjected to an ISO Cytotoxicity Test to measure the level of any toxic residuals that may be remaining following the cleaning process.
LexaMed’s cleaning validation services provide manufacturers with independent confirmation that their processes meet regulatory requirements and safety standards. By ensuring medical devices are effectively cleaned and free of harmful residues they can then be effectively sterilized or disinfected ensuring their safety for repeated use.
Effective cleaning of a reusable device is dependent on the materials of construction and design of the product. In the early stages of product development manufacturers of these devices should consider materials and configurations that will facilitate proper cleaning and eliminate such things as mated surfaces and channels which can present challenges for proper cleaning and allow for unwanted soil accumulation. LexaMed’s project managers and scientists have extensive experience in helping manufacturers design reusable devices in a manner to enhance their cleanability to ensure the safe and effective reprocessing of the device.

February 18, 2025
Hayden Rowan
Medical Device Testing, Pharmaceutical Testing, Lot Release Testing, Turnaround Times
Sterility Assurance, USP <71> Bioburden Determinations, ISO 11737-1, Microbial Limits, USP <61>, USP <62>, Bacterial Endotoxin Testing, Limulus Amebocyte Lysate, LAL, USP <85>, AAMI ST72
At LexaMed, we understand that time is critical. That’s why we pride ourselves on delivering the fastest turnaround times in the industry. Whether it’s sterility, bioburden, or endotoxin testing, we consistently exceed industry standards, ensuring you get the results you need – when you need them. With our advanced technology and expert team, we make speed and accuracy our priority, helping you make quick, informed decisions. Choose LexaMed for all routine testing that works at your pace!
LexaMed’s in-house microbiology and chemistry services ensure independent confirmation of test results that are completed with the highest quality and most expeditious manner. LexaMed understands how important lot release testing is to getting your product to the market and we pride ourselves with our turnaround times.
Sterility testing is critical for pharmaceuticals and medical devices that claim to be sterile or free from viable microorganisms. At LexaMed, we conduct rigorous sterility tests to verify the absence of bacteria, fungi, and other pathogens, ensuring your products meet the highest safety and regulatory standards. Method suitability and routine sterility testing is performed in an ISO Class 5 cleanroom and is consistent with USP ⟨71⟩ and ISO 11737-2 guidelines. If all proper documentation is received and prior to 12pm, sterility testing is put on test the same day as our lab receives it.
The Bacterial Endotoxins Test (BET) is an in vitro assay for detection and quantification of bacterial endotoxins for injectable pharmaceuticals and medical devices with direct or indirect contact to the cardiovascular system, lymphatic system, or cerebrospinal fluid. LexaMed offers accurate, reliable, and fast bacterial endotoxin testing to help you meet industry standards. Inhibition/Enhancement validations and bacterial endotoxin assays are tested in accordance with USP ⟨85⟩/⟨161⟩ and ISO 11737-3.
A Bioburden test determines the total number of living organisms in or on a medical device, container or component. It is performed on any product that requires control and/or monitoring of bioburden counts. LexaMed offers a rapid 10-day turnaround for routine bioburden determination, combining speed with unmatched accuracy. Bioburden recovery validations and routine determinations are performed per ANSI/AAMI/ISO 11737-1.

February 7, 2025
Anne M. Schuler
Microbial stability studies, pharmaceuticals, reconstituted drug products, diluted drug products, microbial contamination, microbial hold time study
USP <51>, Antimicrobial Effectiveness Testing
Pharmaceutical companies must go to great lengths to produce a drug product that is sterile and safe for use. When the sterile product is administered immediately after penetration of the container closure system there is no time for microorganisms which may have entered the during penetration to grow. However, many sterile drug products require reconstitution or dilution in a hospital pharmacy prior to use. These prepared drugs may then be held for a period of time prior to administration during which time microorganisms have an opportunity to grow. Manufacturers of non-preserved drugs, that will not be immediately administered to a patient, must demonstrate that the product will not support microbial growth during the labeled storage period under the stated storage conditions.
ICH Guideline Q8 (R2) Pharmaceutical Development states: “Where relevant, microbial challenge testing under testing conditions that, as far as possible, simulate patient use should be performed during development. Additionally, ICH Guideline Q1A (R2) Stability Testing of New Drug Substances and Products provides the following instruction: “Stability testing of the drug product after constitution or dilution, if applicable, should be conducted to provide information for the labeling on the preparation, storage condition, and in-use period of the constituted or diluted product. This testing should be performed on the constituted or diluted product through the proposed in-use period on primary batches as part of the formal stability studies at initial and final time points”
LexaMed performs Microbial Stability Studies (MSS) to evaluate the microbiological stability of drug products under specified storage conditions following reconstitution and/or dilution with specified diluents to demonstrate the product does not support microbial growth throughout storage in its end-use condition.
Test articles are reconstituted and/or diluted with diluents specified in the product labeling and then inoculated with ≤ 100 CFU/mL of the challenge organisms. Challenge organisms include strains listed in USP <51> Antimicrobial Effectiveness Test specifically, Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Candida albicans, Aspergillus brasiliensis and typical skin flora associated with nosocomial infection. The inoculated test articles are then stored at the storage conditions (temperature and duration) specified in the proposed label. A positive control prepared in the diluent solution and a product negative control (without inoculation) are also included.
Test articles are stored for a period exceeding 2-3 times that of the maximum holding time in the proposed label with samples tested at periodic intermediate timepoints during storage to demonstrate that the diluted product does not support microbial growth for at least the maximum storage period under the specified storage conditions. At each sampling timepoint, surviving viable microorganisms are enumerated using a qualified neutralization method. The change in log10 values of the microbial concentration at each timepoint are calculated and compared to the initial positive control concentration and expressed in terms of log10 change. A test article is considered to have microbial growth if the population is increased by more than 0.5 log10 units compared to the initial value measured. For more information about MSS testing at LexaMed or help with study design please reach out to our subject matter expert, Xiaoqun (Xiao) Zeng by completing the form.
LexaMed’s ISO 13485-2016 Certificate has been updated on our Company Document page. You may also download a copy of it here.
As of March 7, 2024, LexaMed is actively recruiting for the following positions: Microbiologist, Lab technician, and Chemist.
LexaMed’s ISO 17025 Accreditation Certificate has been updated on our Company Document page. You may also download a copy of it here.
LexaMed’s DEA Controlled Substance Registration Certificate has been updated on our Company Document page. You may also download a copy of it here.
LexaMed’s ISO 13485: 2016 Certificate has been updated on our Company Document page.
Based on the current extraordinary events related to COVID-19, SIA Global has granted LexaMed with an extension of our current ISO 13485. The expiration date is now March 31, 2022. Please click here to download our extension letter for your records.
As 2021 comes to completion, we would like to thank all of our clients for the continued trust with all their needs. We look forward to continuing meeting all of you needs in 2022 and beyond.
LexaMed will be following the following schedule for the upcoming holidays.
Christmas:
LexaMed will be closed Thursday, December 23, 2021 at noon and closed all day Friday, December 24, 2021.
New Years:
LexaMed will be closed Friday, December 31, 2021. We will return to normal operating hours (8:30am EST – 5:00 pm EST) on Monday, January 3, 2022.
Copyright @ 2025 LexaMed. All Rights Reserved